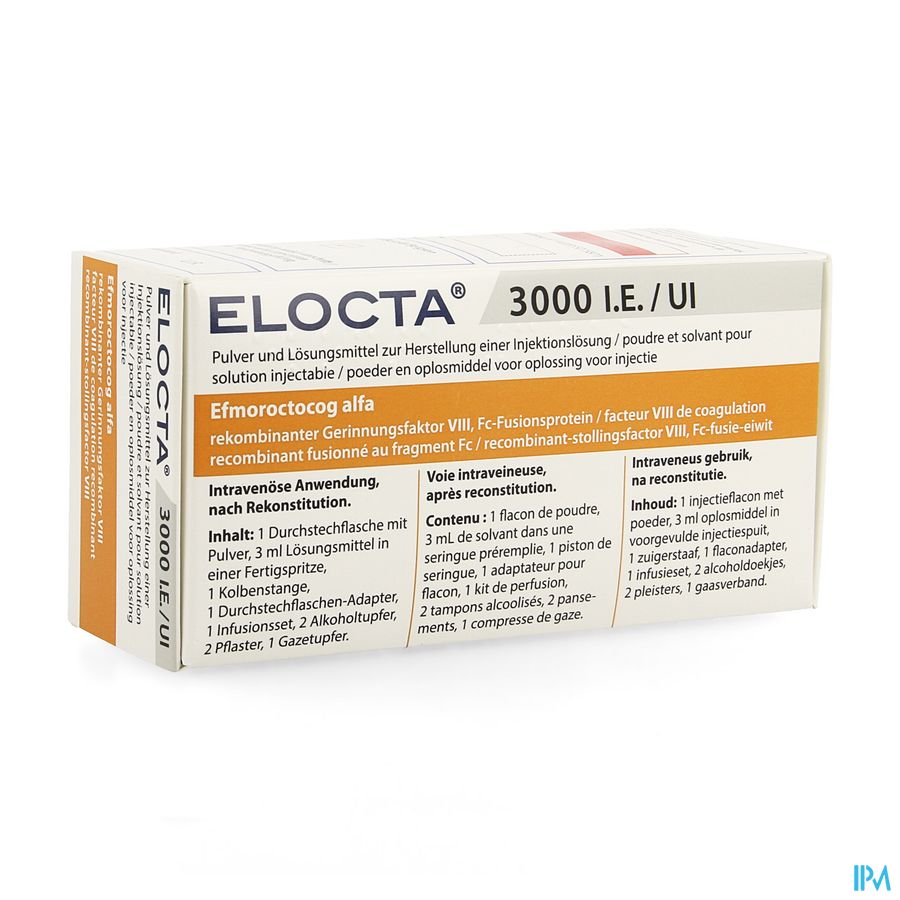
4.8 Undesirable effects Summary of the safety profile Hypersensitivity or allergic reactions (which may include angioedema, burning and stinging at the infusion site, chills, flushing, generalised urticaria, headache, hives, hypotension, lethargy, nausea, restlessness, tachycardia, tightness of the chest, tingling, vomiting, wheezing) have been observed rarely and may in some cases progress to severe anaphylaxis (including shock). Development of neutralising antibodies (inhibitors) may occur in patients with haemophilia A treated with factor VIII, including with ELOCTA. If such inhibitors occur, the condition will manifest itself as an insufficient clinical response. In such cases, it is recommended that a specialised haemophilia centre be contacted. Tabulated list of adverse reactions The Table 2 presented below is according to the MedDRA system organ classification (SOC and Preferred Term Level). Frequencies of adverse reactions are based on clinical studies with a total of 379 patients with severe haemophilia A, of which 276 were previously treated patients (PTPs) and 103 were previously untreated patients (PUPs). See section 5.1 for additional details on the clinical studies. Frequencies have been evaluated according to the following convention: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000), not known (cannot be estimated from the available data). Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness. Table 2: Adverse reactions reported for ELOCTA in clinical trials1 MedDRA System Organ Class Adverse reactions Frequency category1 Blood and lymphatic system disorders FVIII inhibition Uncommon (PTPs)2 Very common (PUPs)2 Nervous system disorders Headache Uncommon Dizziness Uncommon Dysgeusia Uncommon Cardiac disorders Bradycardia Uncommon Vascular disorders Hypertension Uncommon Hot flush Uncommon Angiopathy4 Uncommon Respiratory, thoracic, and mediastinal disorders Cough Uncommon Gastrointestinal disorders Abdominal pain, lower Uncommon Skin and subcutaneous tissue disorders Papular rash Common (PUPs)3 Rash Uncommon Musculoskeletal and connective tissue disorders Arthralgia Uncommon Myalgia Uncommon Back pain Uncommon Joint swelling Uncommon General disorders and administration site conditions Device related thrombosis Common (PUPs)3 Malaise Uncommon Chest pain Uncommon Feeling cold Uncommon Feeling hot Uncommon Injury, poisoning, and procedural complications Procedural hypotension Uncommon PTPs = previously treated patients, PUPs = previously untreated patients. 1 ADRs and frequency are based on occurrence in PTPs only, unless otherwise noted. 2 Frequency is based on studies with all FVIII products which included patients with severe haemophilia A. 3 ADRs and frequency are based on occurrence in PUPs only. 4 Investigator term: vascular pain after injection of ELOCTA.